Describe What Changes Occur During Alpha Decay
Alpha α α rays beta β β rays and gamma γ γ rays. - very light moves at 910th the speed of light - weak ionizing ability but can ionize air molecules -.

Radioactivity Types Of Radioactive Decay Alpha Beta And Gamma Q A
Click Create Assignment to assign this modality to your LMS.
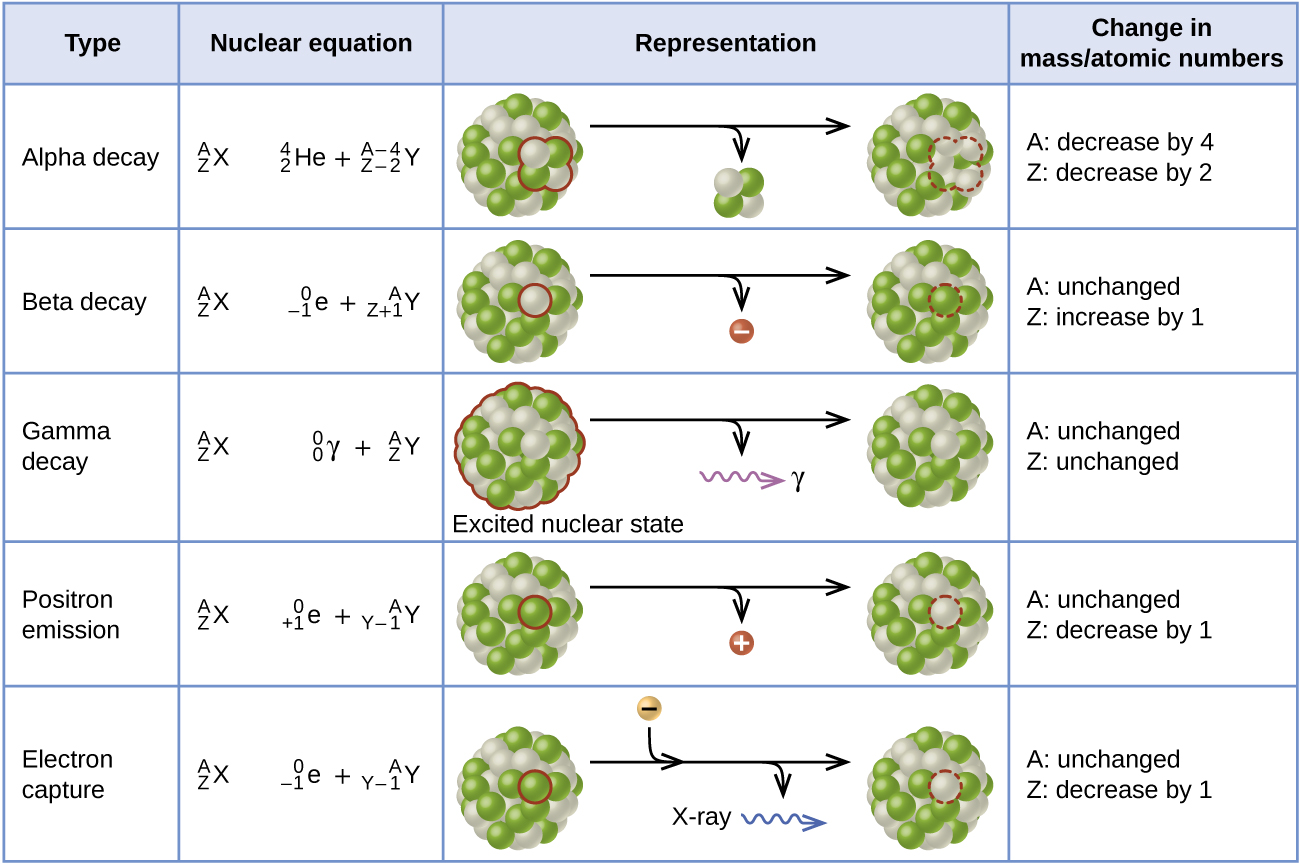
. Alpha decay involves the loss of a helium nucleus beta decay concerns protons turning into neutrons or vice versa and gamma decay involves the emission of energy without changing the original atom. C The mass number is unchanged and the atomic number decreases. C The mass number is unchanged and the atomic number decreases.
Use nuclear symbols to describe changes that occur during nuclear reactions Describe processes involved in the decay series of heavy elements Early experiments revealed three types of nuclear rays or radiation. The mass number is unchanged and the atomic number decreases. Heavy unstable nuclei emit radiation.
Emmiting radiation during the process of radioactive decay. A The mass number and atomic number decreases. -only happens with alpha or beta particles.
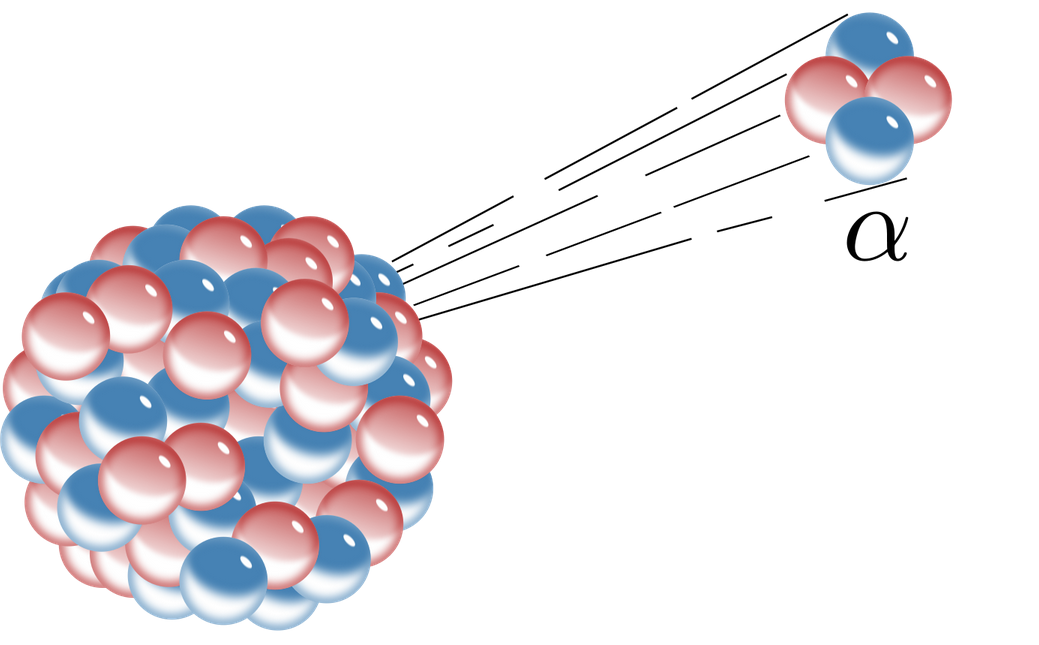
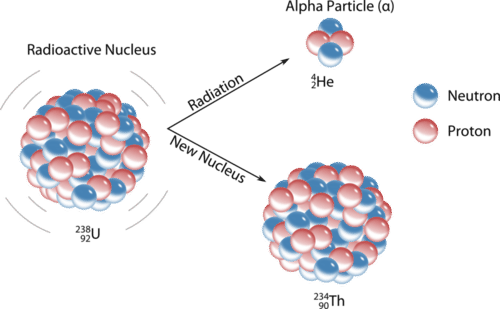
Before the decay the nucleus is called the parent nucleus. - fast moving electron. An alpha particle is a helium-4 nucleus _24He which contains two protons and two neutronsWhen the alpha particle is emitted the atomic number of the atom decreases by two since atomic number is the number of protons which causes the transmutation of the.
A usually irreversible chemical reaction involving the rearrangement of the atoms of one or more substances and a change in their chemical properties or composition covalent bond the bond formed by the sharing of a pair of electrons by two atoms. In this decay a heavier nuclei is converted to lighter nuclei with the release of alpha particle. 19 Describe what changes occur during alpha decay.
Figure 1014 In the Uranium-238 decay series alpha α decays reduce the atomic number as indicated by the red arrows. How is the atomic of the nucleus changed by alpha decay-2. An alpha particle with its two protons and two neutrons is a very stable configuration of particles.
B The mass number and atomic number increase. 2 Points A The mass number and atomic number decreases. The atomic number decreases by 2 and mass number decreases by 4.
B The mass number and atomic number increases. The maximum potential energy of the barrier can be calculated as 30 MeV but alpha particles with energy of 418 MeV are emitted from 238 U by tunnelling as illustrated by Fig. Alpha beta and gamma.
Alpha decay of a nucleus can occur despite the presence of the potential repulsion barrier. E The mass number and atomic number do not change. Gamma - has no charge so not affected.
The mass number and atomic number decreases. The alpha particle is also known as helium atom. A The mass number and atomic number decrease.
Alpha decay or α-decay is a type of radioactive decay in which the atomic nucleus emits an alpha particle thereby transforming or decaying into a new atomic nucleus. How and why alpha decay occurs its dangers and how to write a balanced nuclear equation for alpha decay. There are three major types of radioactive decay.
Here the atomic mass number of the newly formed atom will be reduced by four and the atomic number will be reduced by two. We have a new and improved read on this topic. D The mass number increases and the atomic number decreases.
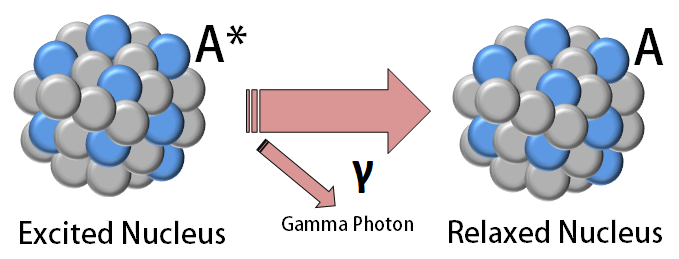
8 Describe what changes occur in the atomic nucleus during gamma ray emission. Since gamma has no charge theres no change in the atomic numbermass number. A The mass number and atomic number decrease.
It only occurs when other decay occurs alphabeta. Alpha particle released carries a mass of 4 units and a charge of 2 units. 3-3 the nucleus emits a 4 He nucleus an alpha particle.
B The mass number and atomic number increases. Notice that for Bi 21 the decay may proceed through either alpha or beta decay. The series ends at the stable nucleus Pb-206.
D The mass number is unchanged and the atomic number increases. Alpha decay beta decay and gamma decay. 23This cannot be explained by classical physics.
The mass number is unchanged and the atomic number increases. Alpha decay involves the emission of an alpha particle by a radioactive isotope of an element. Alpha decay occurs most often in massive nuclei that have too large a proton to neutron ratio.
In -particle decay or alpha decay the nucleus loses two protons and two neutrons so the atomic number decreases by two whereas its mass number decreases by four. 16 Describe what changes occur during gamma ray emission. How is the atomic of the nucleus changed by beta decay 1.
Alpha decay is one of the nuclear decay process. Describe what changes occur during alpha decay. Alpha decay will cause transmutation to occur - this means that one element will turn into another element as the alpha particles are released.
This penetration of the potential barrier is often called tunnelling. In alpha decay shown in Fig. The mass number and atomic number increases.
C The mass number is unchanged and the atomic number decreases. In this decay process alpha particle is released. Alpha particles are He atoms which have had their electrons removed giving them a 2 charge.
In this decay the atomic number of the atom. Beta β decays increase the atomic number as indicated by the blue arrows. A The mass number and atomic number decreases.
The atomic number increases by 1. Solved Describe what changes occur during alpha decaya. Alpha decay is a nuclear change process which produces an alpha particle.
B The mass number and atomic number increase. Alpha radiation reduces the ratio of protons to. The parent nucleus loses 2 protons and 2 neutrons.
3 types of radiation.

Alpha Decay Beta Decay Gamma Decay Electron Capture Positron Production Nuclear Chemistry Youtube

Radioactive Decay Types Article Article Khan Academy

Alpha Decay Explanation Examples Gamow Theory Of Alpha Decay

Introduction To Chemistry Controlling Chemical Reactions Crossword Puzzle Chemical Reactions Crossword Puzzle Chemistry Activities

Alpha Decay An Overview Sciencedirect Topics
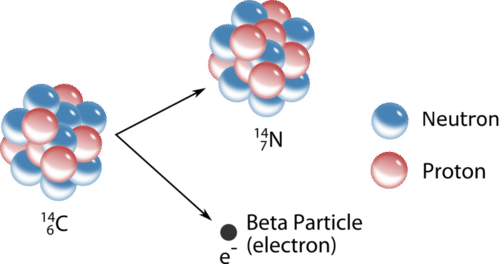
11 4 Nuclear Decay Chemistry Libretexts

Making Predictions About Radioactive Nuclei Decay Video Lesson Transcript Study Com

Alpha Decay An Overview Sciencedirect Topics

Alpha Decay Definition Example Facts Britannica
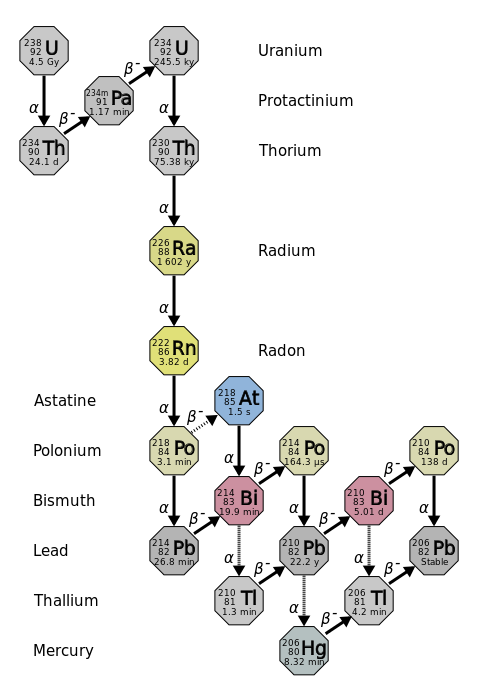.svg.png?revision=1)
17 3 Types Of Radioactivity Alpha Beta And Gamma Decay Chemistry Libretexts

Alpha Decay Explanation Examples Gamow Theory Of Alpha Decay

Alpha Decay Definition Mechanism Types Uses And Examples

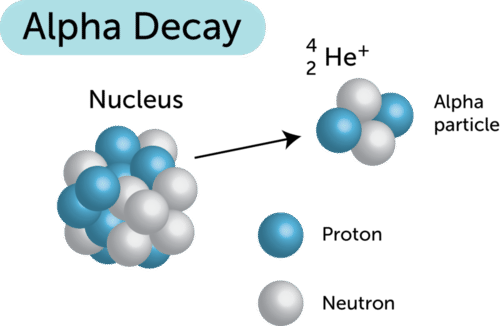

Comments
Post a Comment